Within the Bio-health industry, we develop approved packaging for two classes of materials, which ultimately have the fact in common that they may pose a risk to either people or the environment.
We design and develop approved packaging for the Bio-health industry. We have more than 60 approved references. We offer approved packaging that guarantees maximum protection and safety, adapting to the needs of our customers. We are experts in outer packaging, offering sealed packaging for infectious substances.
Approved packaging for the Bio-Health industry.
The necessary packaging for these substances contains, on the one hand, sealed primary containers (of glass, metal or plastic) that will contain the infectious substance or the diagnostic sample, so the packaging will need a buffer material, plastic secondary packaging or thermal insulation . Additionally, it requires an approved outer packaging in a resistant material such as cardboard, of which Alfilpack is an expert. The sector at which this is aimed:
- Courier services
- Hospitals and laboratories
- Pharmaceutical industry

Examples of Approved Packaging
Box C-010, class 6.1 | Biot-1 box, class 6.2
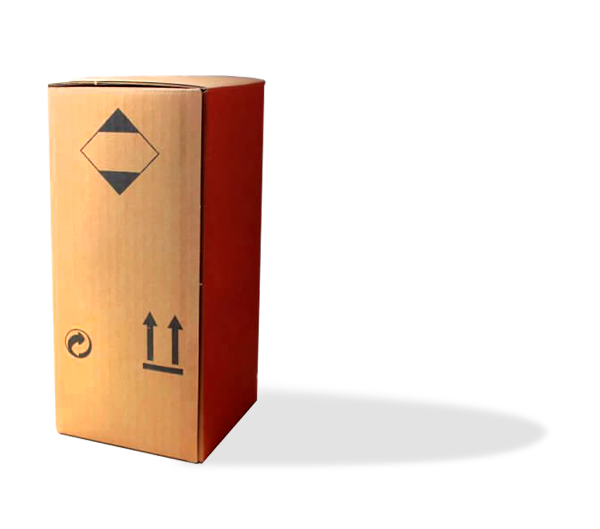
Box C-010, class 6.1
The packaging designed by ADREXPLO consists of Combined Packaging, of:
- Outer Packaging, consisting of a 4G box type 0201
- Inner Container: Paper sheets, case boxes with loose Blister or Blister.
Interior measurements: 785 x 585 x 714 mm
Maximum gross weight: 50kg
Authorised classes: Substances of class 6.1
Applicable regulations: ADR-RID, MDG, ATA-ICAO
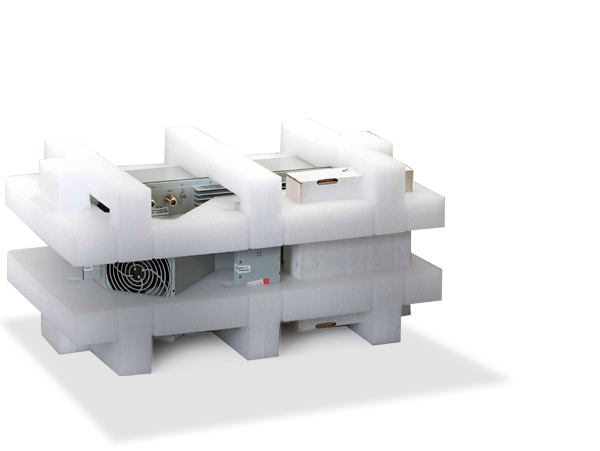
Biot-1 box, class 6.2
The packaging designed by ADREXPLO consists of:
Several primary containers (glass, metal or plastic vials) sealed with a rubber stopper. These primary containers are those that will contain the diagnostic material or infectious material to be transported; therefore they are protected with sufficient buffer material.- Secondary packaging is made of plastic and thermal insulation.
- Absorbent material in sufficient quantity to absorb the entire contents (it is placed between the primary container and the secondary packaging).
- A conditioner that surrounds and protects the secondary packaging.
- An outer packaging of corrugated cardboard of sufficient strength.
Interior measurements: 175 x 175 x 220 mm
Maximum gross weight: See table
Authorised classes: Class 6.2 infectious substances
Applicable regulations: ADR-RID, MDG, ATA-ICAO
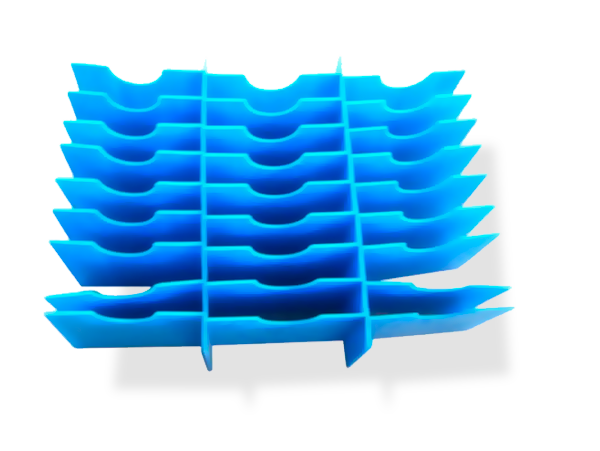
Protections and Positioners
The Packaging Approved for the Bio-health industry is compatible with positioners adapted to the product, in order to guarantee the correct transport of the goods and also to ensure:
*The chosen positioning system follows the ADR regulation that stipulates the conditions
of the packaging entrusted for the transport of dangerous goods.


 Español
Español